|
Chemical
Ionization Mass Spectrometry-Based
Measurements of HO2 and RO2 During TRACE-P
Christopher A. Cantrell, NCAR
Boulder, CO
The
peroxy radical CIMS (PerCIMS) is generally based on the selected
ion CIMS measurement of hydroxyl radicals. In the PerCIMS method,
reagent gases NO and SO2 are added to ambient air, leading
to the conversion of ambient peroxy radicals (RO2 and/or
HO2) to gas-phase sulfuric acid.The H2SO4 product
is detected first by ionization through a proton transfer reaction
with , followed by introduction
into a vacuum system with ion optics, differential pumping, quadrupole
mass filter and channel electron multiplier for ion
counting.
The PerCIMS instrument
is unique in that we have developed methods to yield measurement
of total peroxy
radical levels ([HO2] + [RO2]), and measurement of [HO2]
by adjusting the reagent gas concentrations in the inlet region. These
two measurements yield additional information about atmospheric processing of
hydrocarbons and the branched chain reactions that lead to partitioning of peroxy
radicals between the hydro- and organic-peroxy radical
forms.
Calibration of
the instrument is accomplished through photolysis of water vapor
using 184.9 nm radiation from
a low-pressure mercury lamp. Radical
concentrations in the range of ambient concentrations (and higher) are easily
generated by adjusting the lamp distance, slit width and water vapor concentration.
The lamp flux is determined through N2O actinometry. Relative
sensitivity of various peroxy radicals are measured using the
photolysis of Cl2 to yield chlorine atoms that react with hydrogen
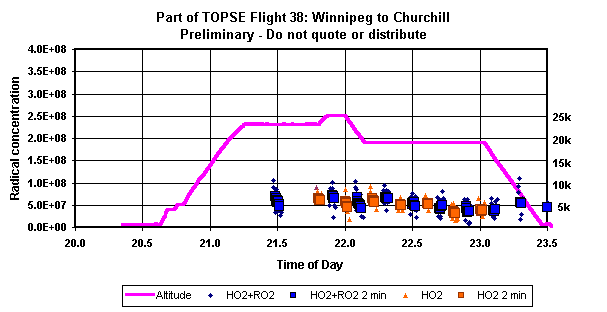
and various hydrocarbon precursors. Sample
data from the TOPSE aircraft campaign are shown in the figure.
These
measurements are accomplished with an estimated ±30%
accuracy, detection limit of 1 x 107 radicals-cm-3 in
15 seconds, and typical 2s
precision of 10% at 1 x 108 radicals-cm-3. Lower
detection limits with better precision are possible with
appropriate averaging.
|